RESEARCH
- HOME
- RESEARCH
Cardiomyocyte dedifferentiation and proliferation mechanisms
In contrast to mammals, the teleost zebrafish exhibits remarkable regenerative capacity of multiple adult organs, including the heart. We and others previously demonstrated that cardiomyocyte dedifferentiation and proliferation, but not de novo differentiation from stem cells, is the crucial mechanism for new muscle regeneration in the zebrafish heart. Notably, the same regenerative mechanism has been discovered in neonatal mammals; however, the self-renewal capacity of cardiomyocytes rapidly diminishes after birth. We harness the advantages of both zebrafish and mice to expand our understanding of the fundamental cardiomyocyte dedifferentiation and proliferation mechanisms, thereby providing novel insights for future regenerative therapies of the human heart.
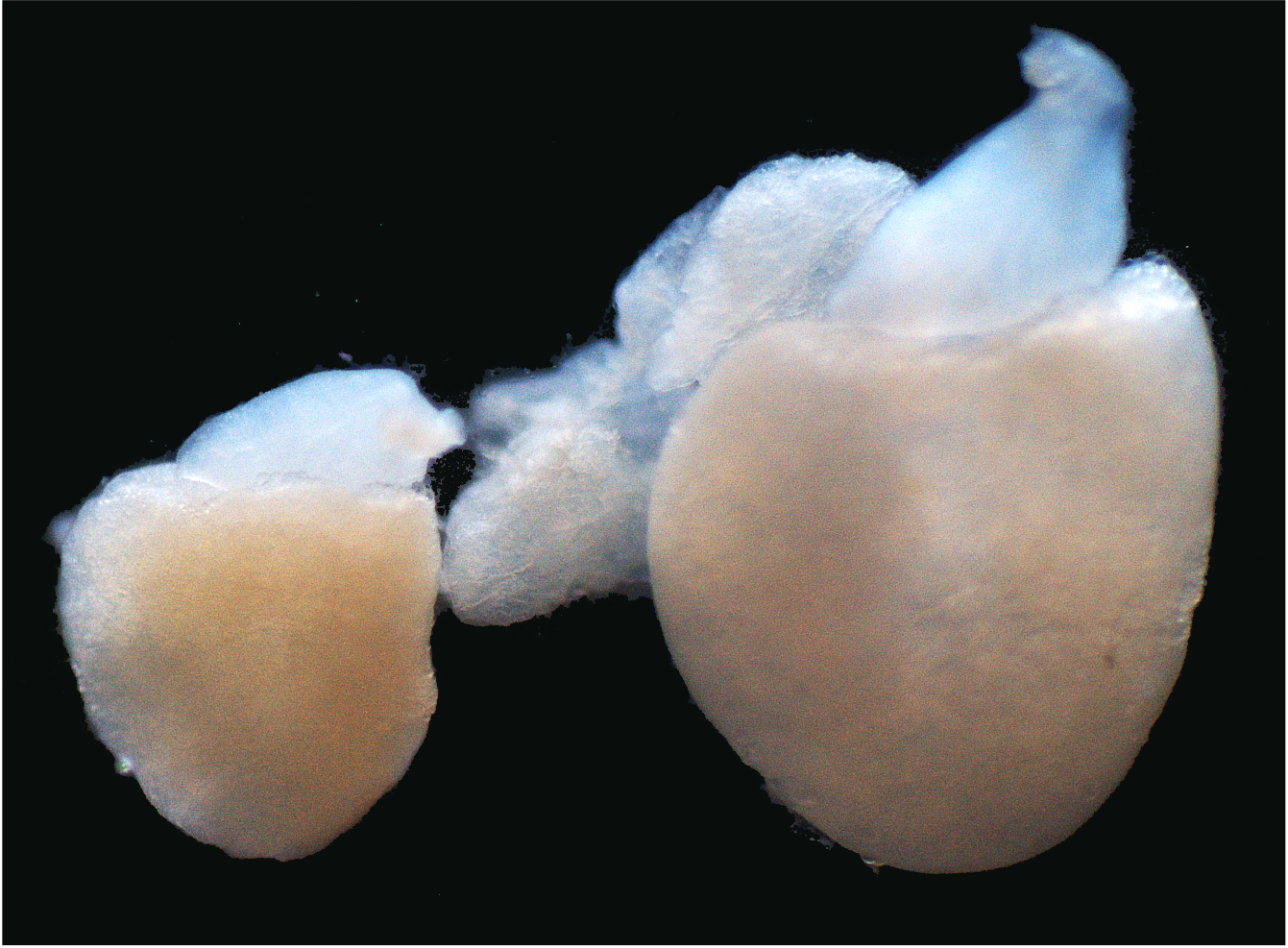
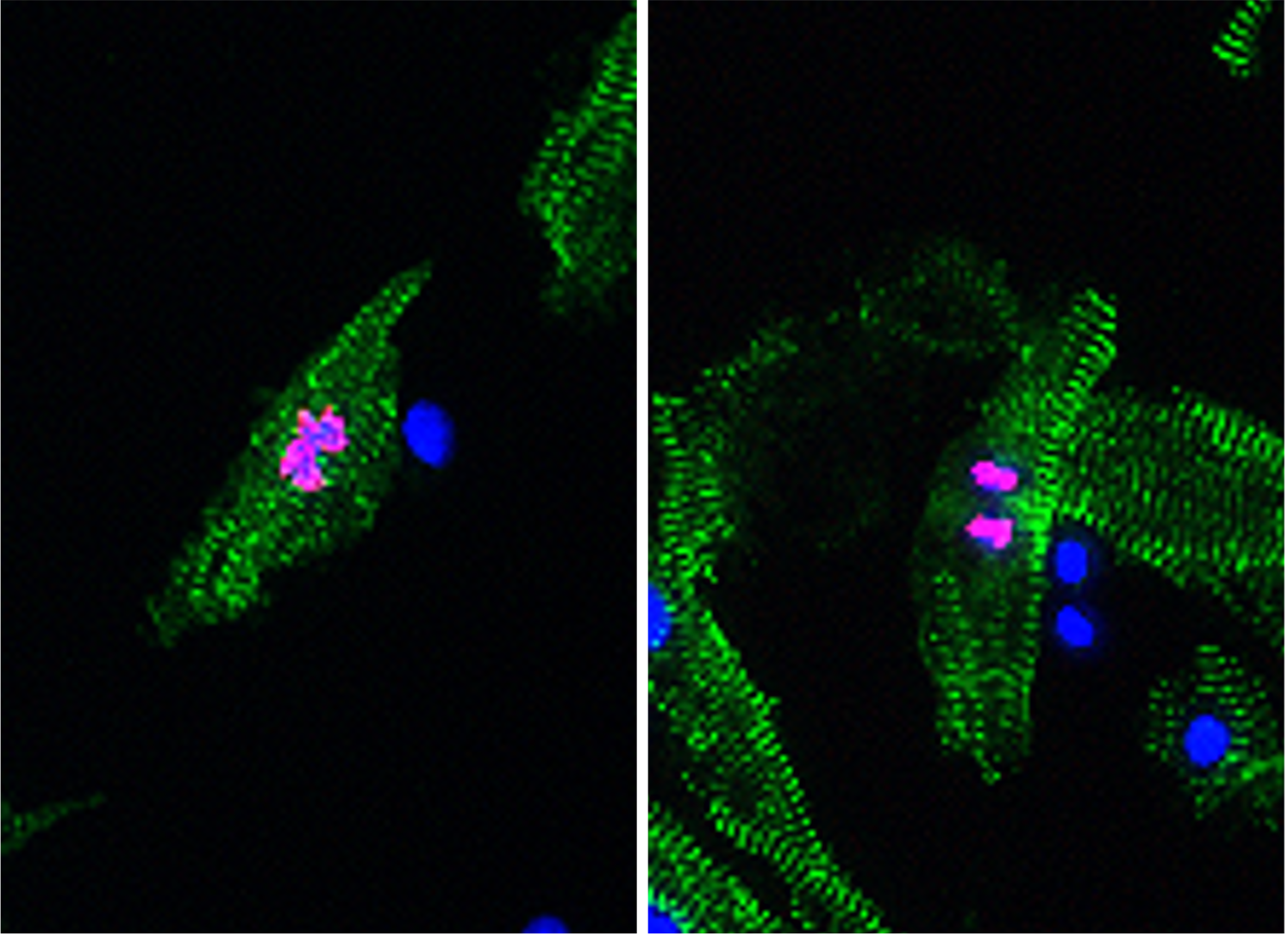

Cardiomyocyte renewal therapy
We recently discovered that KLF1, a member of the Krüppel-like transcription factors, while being pivotal for red blood cell development and congenital anemia, also drives an entirely novel cardiomyocyte dedifferentiation and proliferation mechanism in zebrafish. Our results suggest that KLF1 expression modifications could reactivate cardiomyocyte proliferative capacity in adult mammalian hearts. Currently, we are developing KLF1-mRNA therapies focusing on cardiomyocyte renewal to induce better repair in damaged human hearts.
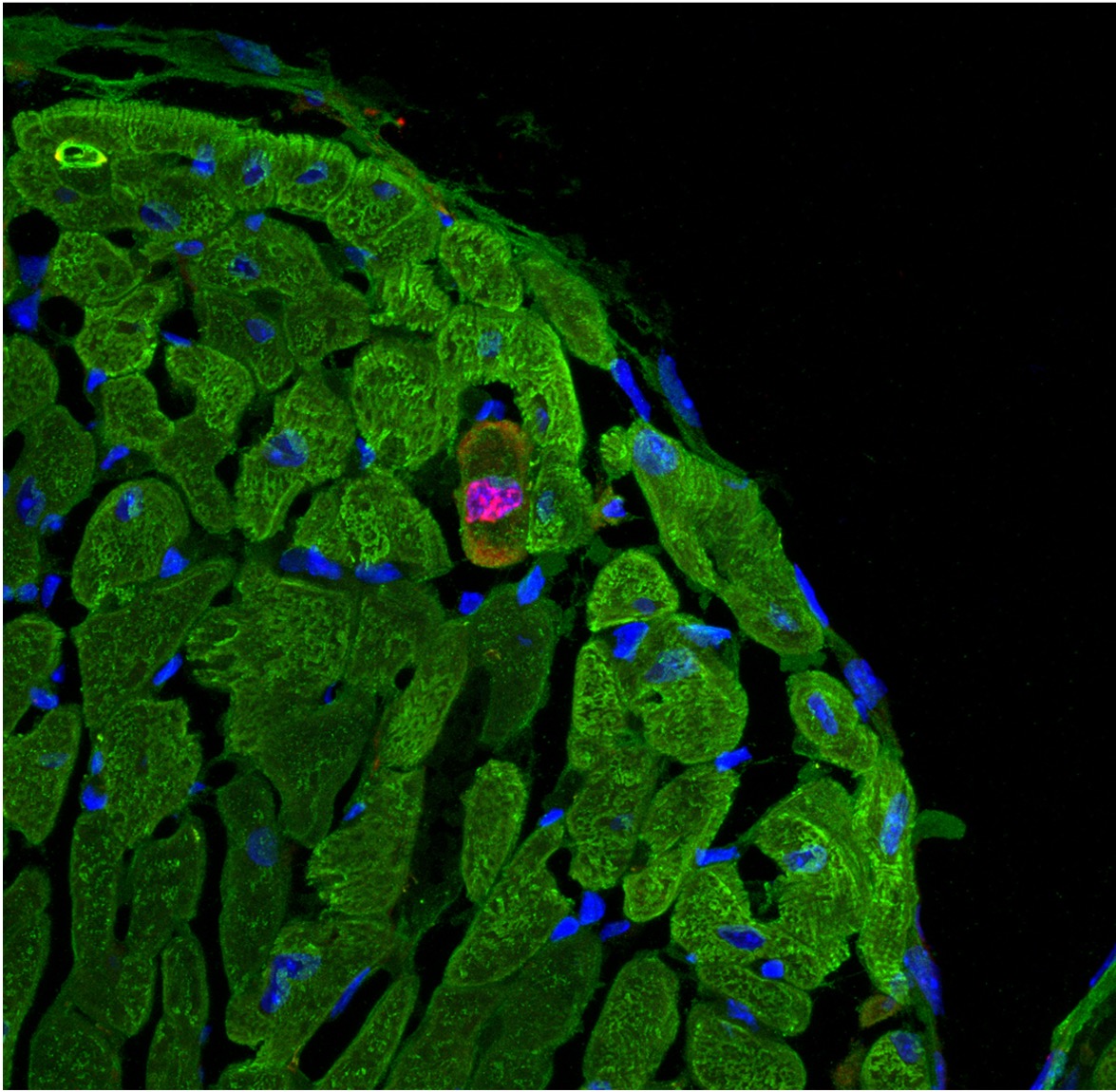
Live imaging of tissue homeostasis and regeneration
As another ongoing project, we aim at establishing Danionella cerebrum as a novel model organism for live imaging of the adult vertebrate cardiovascular system. Danionella is a genetically tractable freshwater minnow with notable transparency throughout its lifetime and a potentially useful in vivo imaging model of cell signaling and cellular networks in adult tissues. Live imaging analysis of danionella would help us gain fundamental insights into intercellular signaling and cellular event coordination in cardiovascular homeostasis, regeneration, and diseases.
